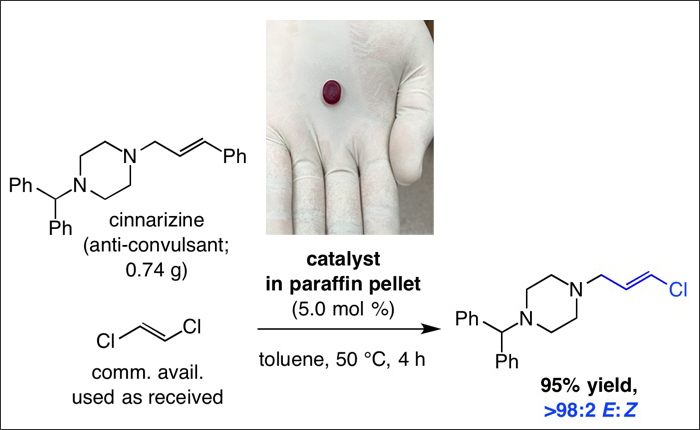
A significant shortcoming in olefin metathesis, a chemical process that is central to research in several branches of chemistry, is the complete lack of efficient methods that kinetically favor E-isomers in the product distribution. Now a team of chemists report in a recent issue of the journal Science (2016, 352, 569–575) that kinetically E-selective cross-metathesis reactions may be designed to generate thermodynamically disfavored alkenyl chlorides and fluorides in high yield and with exceptional stereoselectivity. The researchers demonstrate that with 1.0–5.0 mol % of a molybdenum-based catalyst, which may be delivered in the form of air- and moisture-stable paraffin pills soon to be commercially available by Aspira, Inc., reactions typically proceed to completion within four hours at ambient temperature. Many isomerically pure E-alkenyl chlorides, applicable to catalytic cross-coupling transformations and found in biologically active entities, thus become easily and directly accessible. Similarly, E-alkenyl fluorides can be synthesized from simpler compounds or more complex molecules.
See the recent publication by Richard Schrock and Amir Hoveyda:
Kinetically Controlled E-Selective Catalytic Olefin Metathesis