A hallmark of Ru-based olefin metathesis catalysts, which have been pivotal to the emergence of this central transformation, is their ability to promote reactions of hindered alkenes and in the presence of commonly occurring polar functional units such as a neighboring alcohol, an aldehyde, or a carboxylic acid. In 2011, Professor Grubbs and co-workers at California Institute of Technology reported that kinetic control of Z-selectivity can be achieved with Ru catalysts by substituting anionic chlorides with an alkyl and an oxo ligand. In 2013, Professor Amir Hoveyda and co-workers outlined the design of a distinct set of Ru-based complexes that contain a key a bidentate disulfide ligand and may be used for efficient catalytic Z-selective olefin metathesis. The CalTech catalysts have proven to be inefficient in promoting transformations with aryl-substituted or hindered terminal olefins and/or when an aldehyde is present, and their activity is entirely inhibited by a carboxylic acid. The BC catalysts, on the other hand, tolerate such functional groups. Nonetheless, the derived methylidene species decompose too rapidly and reactions involving terminal alkenes, the most accessible substrate class, are low yielding.
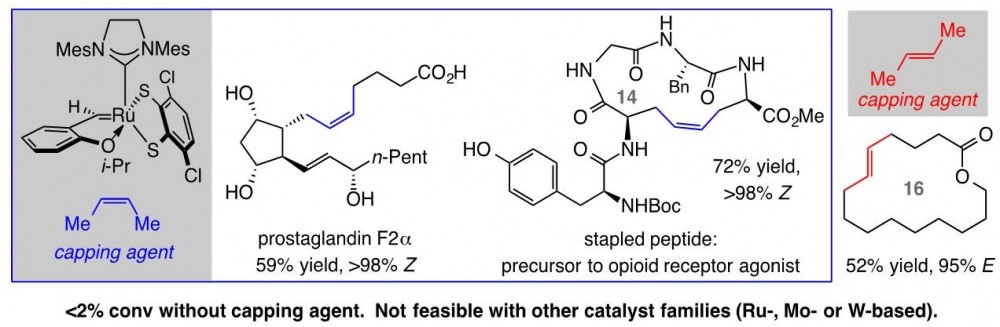
In a paper that just appeared in the Journal of the American Chemical Society (DOI:10.1021/jacs.7b06552), Professor Hoveyda and graduate student Chaofan Xu and postdoctoral fellow Dr. Xiao Shen demonstrate that by adding Z-butene to a reaction mixture that contains an appropriate Ru dithiolate catalyst, methylidene complex formation can be avoided, and, as a result, an unprecedented range of terminal alkenes may be converted to the desired products by efficient and exceptionally Z- or E-selective cross-metathesis and macrocyclic ring-closing metathesis processes. The BC group reports that stereoselectivity is generally higher when the methylene capping strategy is adopted. The broad applicability and general utility of the approach is highlighted by concise (i.e., little or no protection/deptrotection sequences) and stereoselective syntheses of a platelet aggregate inhibitor, prostaglandins E2 and F2alpha as well as a fourteen-membered ring stapled peptide that is precursor to a potent opioid receptor agonist.
See the latest publication by Amir Hoveyda: